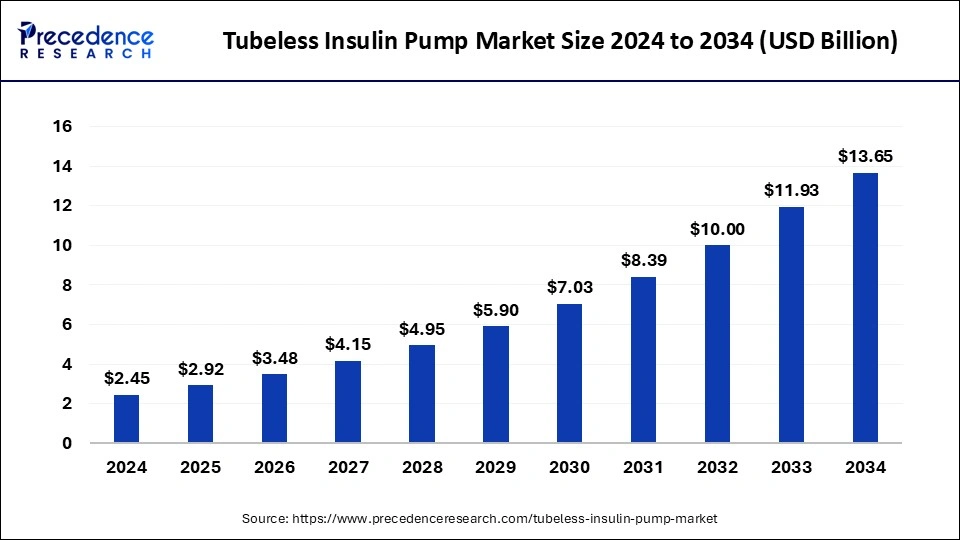
The global tubeless insulin pump market size reached USD 2.05 billion in 2023 and is anticipated to hit around USD 11.93 billion by 2033, growing at a CAGR of 19.25% from 2024 to 2033.
Key Takeaways
- North America held the largest market share of 55% in 2023.
- Asia Pacific is expected to grow at the fastest rate during the forecast period.
- By type, the insulin patch pump segment accounted for the dominating share in 2023. The segment is observed to continue growth at a significant rate in the upcoming period.
- By component, the pod or patch segment held the largest share of the market in 2023.
- By end users, the hospitals segment held the largest share of the market in 2023.
The tubeless insulin pump market has witnessed significant growth in recent years, driven by the increasing prevalence of diabetes globally. Tubeless insulin pumps offer a convenient and discreet way for diabetic patients to manage their insulin delivery, compared to traditional insulin injection methods. These pumps are designed to deliver precise doses of insulin continuously throughout the day, mimicking the function of a healthy pancreas. With advancements in technology and improvements in pump design, the tubeless insulin pump market is poised for continued expansion in the coming years.
Get a Sample: https://www.precedenceresearch.com/sample/3944
Growth Factors:
Several factors contribute to the growth of the tubeless insulin pump market. Firstly, the rising incidence of diabetes, particularly type 1 diabetes, has created a substantial demand for innovative insulin delivery systems. Tubeless insulin pumps provide diabetic patients with greater freedom and flexibility in managing their condition, leading to increased adoption rates. Additionally, technological advancements in pump design have resulted in smaller, more user-friendly devices that offer enhanced comfort and convenience. Moreover, the growing preference for non-invasive insulin delivery methods among patients has further fueled the demand for tubeless insulin pumps.
Drivers:
Several drivers are propelling the growth of the tubeless insulin pump market. One of the primary drivers is the increasing awareness about the benefits of continuous glucose monitoring (CGM) and insulin pump therapy among healthcare professionals and patients alike. CGM integration with tubeless insulin pumps enables real-time monitoring of blood glucose levels and allows for more precise insulin dosing, leading to improved glycemic control and better patient outcomes. Furthermore, the convenience and discretion offered by tubeless insulin pumps appeal to patients seeking an alternative to traditional insulin injection methods, driving adoption rates.
Restraints:
Despite the promising growth prospects, the tubeless insulin pump market faces certain restraints that may impede its expansion. One such restraint is the high cost associated with tubeless insulin pump therapy, which may limit access for some patients, particularly in developing regions with limited healthcare resources. Additionally, concerns regarding pump reliability and durability, as well as the need for regular maintenance and troubleshooting, may deter potential users from adopting tubeless insulin pump therapy. Moreover, reimbursement challenges and regulatory hurdles in certain markets pose significant barriers to market growth.
Opportunities:
Despite the challenges, the tubeless insulin pump market presents several opportunities for growth and innovation. The ongoing development of advanced pump technologies, including closed-loop systems and artificial intelligence algorithms, holds promise for improving insulin delivery accuracy and enhancing patient convenience. Moreover, expanding market penetration in emerging economies with large diabetic populations presents a lucrative opportunity for manufacturers to capitalize on untapped market potential. Additionally, collaborations between healthcare providers, technology companies, and research institutions can drive innovation and foster the development of next-generation tubeless insulin pump solutions.
Region Insights:
The tubeless insulin pump market exhibits varying dynamics across different regions. In North America, the market is driven by high diabetes prevalence rates, well-established healthcare infrastructure, and strong adoption of technological innovations. Europe also represents a significant market for tubeless insulin pumps, owing to favorable reimbursement policies, increasing diabetes awareness, and a growing aging population. In the Asia-Pacific region, rapid urbanization, sedentary lifestyles, and changing dietary habits are contributing to a surge in diabetes cases, creating opportunities for market expansion. Similarly, Latin America and the Middle East & Africa are witnessing steady growth in demand for tubeless insulin pumps, driven by improving healthcare access and rising diabetes awareness campaigns. Overall, the tubeless insulin pump market is poised for continued growth across diverse geographical regions, fueled by evolving patient needs and advancements in technology.
Recent Developments
- In August 2023, the Tubeless insulin pump received FDA clearance for diabetes people. The Accu-Chek Solo micropump (Roche Diabetes) was granted 510(k) clearance from the FDA. Accu-Chek Solo micropump is tubeless, small, and lightweight. Users can place the device on four different infusion sites on the body. The device is detachable, allowing people with diabetes to change the infusion site when necessary.
- In July 2023, the FDA announced the clearance of Tandem Diabetes Care’s Mobi durable automated insulin pump. The approval covers people with diabetes aged six and above, expanding Tandem's portfolio of products. Mobi features a 200-unit insulin cartridge and an on-pump button to provide an alternative to phone control for insulin boluses.
- In February 2024, Omnipod 5 received approval to integrate with the new freestyle libre two plus sensor. The Omnipod 5 hybrid closed loop system has received approval to integrate with the Abbott FreeStyle Libre 2 Plus Sensor in the UK, which will give people living with diabetes additional choice and flexibility for managing their blood sugar levels.
- In April 2023, Insulet announced FDA clearance of Omnipod GO, a first-of-its-kind basal-only insulin pod for people with type 2 diabetes aged 18 and above. Omnipod GO is a wearable, standalone insulin delivery system that provides a fixed rate of continuous rapid-acting insulin for 72 hours.
Tubeless Insulin Pump Market Companies
- Medtronic plc
- Hoffmann-La Roche Ltd
- Tandem Diabetic Care, Inc.
- Insulet Corporation
- Ypsomed
- Cellenovo
- Abbott
- Tandem Diabetes Care
- Insulet Corporation
- Sooil Development
- Valeritas, Inc
- JingasuDelfu Co., Ltd.
- Cellnova
- Roche Holdings
- Spring Health Solutions
- Johnson & Johnson
- Medtrum Technologies
- Debiotech
- CeQur
- Valeritas Holding
- Animas Corporation
Segments Covered in the Report
By Type
- Insulin Patch Pump
- Traditional Pump
By Component
- Pod or Patch
- Remote
- Accessories
By End-users
- Hospitals
- Pharmacies
- E-commerce
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/


0 Comments