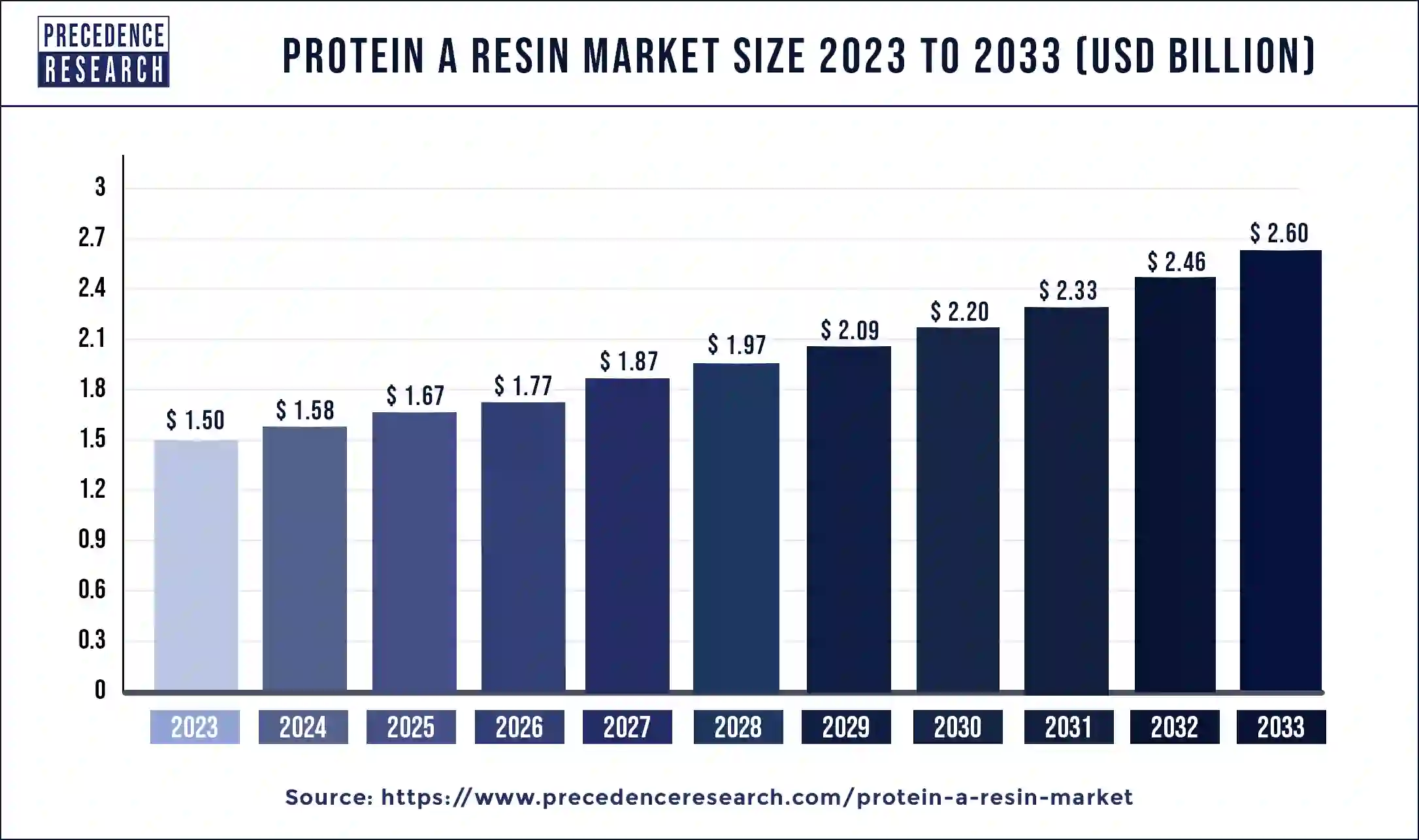
The global protein A resins market size was estimated at USD 1.50 billion in 2023 and is projected to cross around USD 2.60 billion by 2033, growing at a CAGR of 5.65% from 2024 to 2033.
Key Takeaways
- By region, North America led the protein A resins market with the largest market share of 42% in 2023.
- By region, Asia Pacific is expected to witness the fastest growth rate in the market during the forecast period.
- By application, the antibody purification segment dominated the protein A resins market in 2023 with market share of 60%.
- By product, the recombinant protein A segment had the largest share of around 58% in 20223.
- By matrix type, the agarose-based matrix segment dominated the market in 2023 with market share of 42.3%.
- By end-user, the pharmaceutical & biopharmaceutical companies segment dominated the market in 2023 with market share of 59%.
Protein A resins play a pivotal role in the biopharmaceutical industry, serving as crucial tools for the purification of monoclonal antibodies (mAbs) and other therapeutic proteins. The global market for Protein A resins is witnessing significant growth, driven by several key factors.
Get a Sample: https://www.precedenceresearch.com/sample/4063
Growth Factors:
One of the primary growth drivers for the Protein A resins market is the increasing demand for monoclonal antibodies (mAbs) and other biopharmaceuticals. With the rising prevalence of chronic diseases and the growing aging population, there is a heightened need for innovative therapeutic solutions, driving the production of mAbs and other biologics.
Moreover, advancements in bioprocessing technologies and the adoption of single-use systems are fueling the demand for Protein A resins. These resins offer high binding capacity, selectivity, and efficiency, enabling biopharmaceutical manufacturers to achieve high yields and purity levels in the purification process.
Additionally, the expansion of the biopharmaceutical industry in emerging markets, coupled with investments in healthcare infrastructure, is driving the demand for Protein A resins. Countries in Asia-Pacific, Latin America, and the Middle East are witnessing rapid growth in biologics manufacturing, creating lucrative opportunities for Protein A resin suppliers.
Region Insights:
The Protein A resins market exhibits regional variations driven by factors such as regulatory environment, healthcare infrastructure, and biopharmaceutical manufacturing capacity.
North America dominates the global Protein A resins market, owing to the presence of a well-established biopharmaceutical industry, robust R&D infrastructure, and favorable regulatory policies. The United States, in particular, accounts for a significant share of the market, supported by the presence of major biopharmaceutical companies and contract manufacturing organizations (CMOs).
Europe is another prominent market for Protein A resins, driven by the presence of key biopharmaceutical hubs in countries like Germany, Switzerland, and the United Kingdom. The region benefits from a skilled workforce, strong academic-industry collaborations, and government initiatives to promote biotechnology innovation.
Asia-Pacific is poised to witness significant growth in the Protein A resins market, fueled by the expansion of biopharmaceutical manufacturing in countries like China, India, and South Korea. Rising investments in healthcare infrastructure, increasing R&D expenditure, and favorable government policies are driving the growth of the market in the region.
Protein A Resins Market Dynamics
Drivers:
Several drivers contribute to the growth of the Protein A resins market. The increasing prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases is driving the demand for biopharmaceuticals, including monoclonal antibodies (mAbs). Protein A resins play a critical role in the purification of mAbs, ensuring high product quality and efficacy.
Furthermore, the growing adoption of personalized medicine and targeted therapies is driving the demand for biologics, creating opportunities for Protein A resin manufacturers. These resins enable the efficient purification of therapeutic proteins, supporting the development of novel biopharmaceuticals for personalized treatment approaches.
Moreover, technological advancements in Protein A resin manufacturing, such as the development of novel ligands, improved chromatography media, and high-throughput purification systems, are driving innovation in the market. Manufacturers are continuously investing in research and development to enhance the performance, efficiency, and cost-effectiveness of Protein A resins.
Opportunities:
The Protein A resins market presents several opportunities for growth and expansion. One such opportunity lies in the development of next-generation Protein A resins with enhanced binding capacity, selectivity, and stability. Manufacturers are investing in research and development to develop novel ligands and chromatography media that offer improved performance and cost-effectiveness.
Additionally, the expansion of biopharmaceutical manufacturing capacity in emerging markets presents lucrative opportunities for Protein A resin suppliers. Countries in Asia-Pacific, Latin America, and the Middle East are investing in biotechnology infrastructure and talent development to support the growth of the biopharmaceutical industry, creating a growing demand for Protein A resins.
Furthermore, the increasing adoption of single-use bioprocessing systems presents opportunities for Protein A resin manufacturers to expand their market reach. Single-use systems offer several advantages, including reduced risk of cross-contamination, lower capital investment, and faster turnaround times, driving their adoption in biopharmaceutical manufacturing processes.
Challenges:
Despite the growth opportunities, the Protein A resins market faces several challenges that may impede market growth. One such challenge is the high cost of Protein A resins, which can significantly impact the overall cost of biopharmaceutical production. Manufacturers are continuously seeking ways to reduce production costs and improve the cost-effectiveness of Protein A resins through process optimization and technological innovation.
Moreover, the limited availability of raw materials and the complex manufacturing process pose challenges to Protein A resin suppliers. The production of Protein A resins requires specialized expertise, infrastructure, and quality control measures to ensure product consistency and purity, which can be challenging for small and medium-sized manufacturers.
Additionally, regulatory requirements and quality standards for biopharmaceutical manufacturing pose challenges to Protein A resin suppliers. Manufacturers must comply with stringent regulatory guidelines, including Good Manufacturing Practices (GMP), to ensure the safety, efficacy, and quality of biopharmaceutical products, adding complexity and cost to the manufacturing process.
Recent Developments
- In March 2024, Ultomiris received an approval from the United States for the long-term C5 complement inhibitor for the diagnostics of geriatric patients having anti-aquaporin-4 (AQP4) antibody-positive (Ab+) neuromyelitis optica spectrum disorder.
- In March 2024, Merck, a leading player in the life science technology company expanded its M LabTM Collaboration Center in Shanghai, China, it is the organization’s biggest in the global network of 10 interconnected labs. The investment worth € 14 million was added for the new biology application lab an upstream application lab and a process development training center to its M LabTM Collaboration Center in Shanghai.
- In April 2024, BioArctic AB collaborated with Eisai and announced the organization submitted the supplemental Biologics License Application (sBLA) for its drug Leqembi to the United States Food and Drug Administration (FDA).
- In April 2024, the US FDA approved and authorized the latest antibody drug named Pemgarda marketed by the biotech Invivyd, to protect immunocompromised individuals from COVID-19.
- In April 2024, Kemp Proteins LLC, a leading company in gene-to-protein services and monoclonal antibody development announced the strategic collaboration with the Columbia Biosciences of Frederick, Maryland.
- In April 2024, Sino Biological introduced a series of in vitro bioassay services for maintaining antibody drug development projects. Sino Biological offers different reagents and a range of n vitro efficacy evaluation services for meeting the demand for testing practices and trends.
Protein A Resins Market Companies
- GE Healthcare
- Merck Millipore
- PerkinElmer, Inc.
- GenScript Biotech Corp.
- Agilent Technologies
- Repligen Corp.
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories, Inc.
- Abcam PLC.
- Novasep Holdings SAS
Segments Covered in the Report
By Application
- Antibody purification
- Immunoprecipitation
By Product
- Recombinant protein A
- Natural protein A
By Matrix Type
- Agarose-based matrix
- Glass or silica gel-based matrix
- Organic polymer-based matrix
By End-user
- Pharmaceutical & Biopharmaceutical Companies
- Clinical research laboratories
- Academic research institutes
- Contract research organization
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/


0 Comments