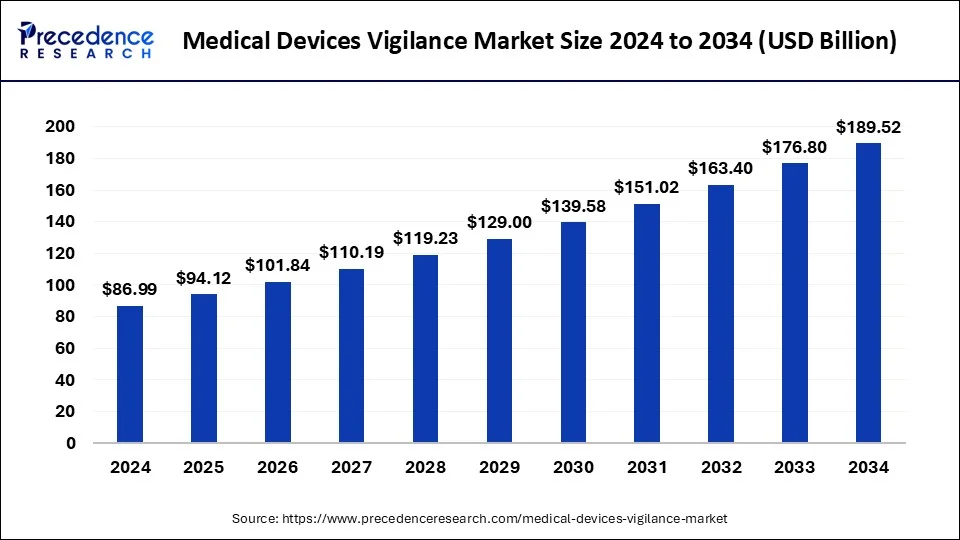
The global medical devices vigilance market size surpassed USD 80.40 billion in 2023 and is projected to hit around USD 176.80 billion by 2033, growing at a CAGR of 8.19% from 2024 to 2033.
Medical Devices Vigilance Market Key Takeaways
- The North America medical devices vigilance market size reached USD 24.12 billion in 2023 and is expected to attain around USD 53.04 billion by 2033.
- North America was estimated to hold a substantial market share of 34% in 2023.
- Asia Pacific is projected to witness rapid growth in the global market.
- By delivery mode, the on-demand segment accounted for the largest share of 81% in 2023.
- By delivery mode, the on-premises segment is expected to have steady growth over the forecast period.
- By application, the diagnostics segment held a substantial market share of 36% in 2023.
- By application, the research segment is expected to show lucrative growth over the forecast period.
- By end use, the clinical research organization segment held the highest market share of 42% in 2023.
- By end use, the business process outsourcing firms segment is expected to grow rapidly in the foreseeable period.
The Medical Devices Vigilance Market is a sector focused on the monitoring and assessment of medical devices for safety and efficacy. This includes the identification, reporting, and analysis of adverse events and potential risks associated with medical devices. The market encompasses regulatory requirements, surveillance systems, and post-market monitoring to ensure patient safety and regulatory compliance.
Growth Factors
The growth of the Medical Devices Vigilance Market is driven by the increasing use of medical devices worldwide and the need for stringent safety standards. As the healthcare industry continues to innovate and introduce new devices, the demand for effective vigilance systems to monitor these products also rises. Additionally, an aging population and the increasing prevalence of chronic diseases contribute to the growth of the market as more medical devices are used in healthcare.
Get a Sample: https://www.precedenceresearch.com/sample/4120
Region Insights
The Medical Devices Vigilance Market sees varying levels of growth across different regions due to differences in regulatory environments and healthcare infrastructure. North America, particularly the United States, leads the market due to its well-established regulatory framework and high adoption of advanced medical technologies. Europe is also a significant market, with strong regulatory oversight and a focus on patient safety. Emerging markets in Asia-Pacific, such as China and India, are witnessing growth due to increasing healthcare expenditure and the adoption of international standards.
Drivers
Key drivers of the Medical Devices Vigilance Market include:
- Regulatory Requirements: Stricter regulations for medical device safety and reporting of adverse events drive the need for effective vigilance systems.
- Technological Advancements: Advances in digital health technologies enable better data collection and analysis for monitoring medical devices.
- Rising Healthcare Expenditure: Increased investment in healthcare infrastructure and technologies supports the adoption of vigilance systems.
Opportunities
Opportunities in the Medical Devices Vigilance Market include:
- Innovation in Monitoring Systems: Developing advanced monitoring tools, such as AI and machine learning, can enhance vigilance capabilities.
- Global Expansion: Expanding vigilance systems into emerging markets presents growth opportunities as healthcare infrastructure improves.
- Collaboration and Partnerships: Collaborations between medical device manufacturers, regulatory bodies, and healthcare providers can lead to improved vigilance practices.
Challenges
Challenges facing the Medical Devices Vigilance Market include:
- Compliance and Standardization: Ensuring compliance with varying international regulations and standards can be complex for global companies.
- Data Management: Managing large volumes of data and ensuring data accuracy and security is challenging.
- Cost of Implementation: The cost of implementing and maintaining effective vigilance systems can be a barrier for some organizations.
Medical Devices Vigilance Market Recent Developments
- In June 2022, Italy instituted substantial changes in national regulations on medical device vigilance in accordance with the procedures by European Regulations. 2017/475 for medical devices and 2017/476 for in vitro diagnostics.
Medical Devices Vigilance Market Companies
- ZEINCRO
- AssurX Inc.
- Sparta System
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd.
- MDI Consultants, Inc.
- AB-Cube
- Laerdal Medical.
- Omnify Software, Inc.
Segments Covered in the Report
By Delivery Mode
- On-demand
- On-premise
By Application
- Diagnostics
- Therapeutics
- Surgical
- Research
By End-user
- Clinical Research Organizations (CROs)
- Business Process Outsourcing (BPO)
- Original Equipment Manufacturers (OEM)
- Other End-users
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/


0 Comments