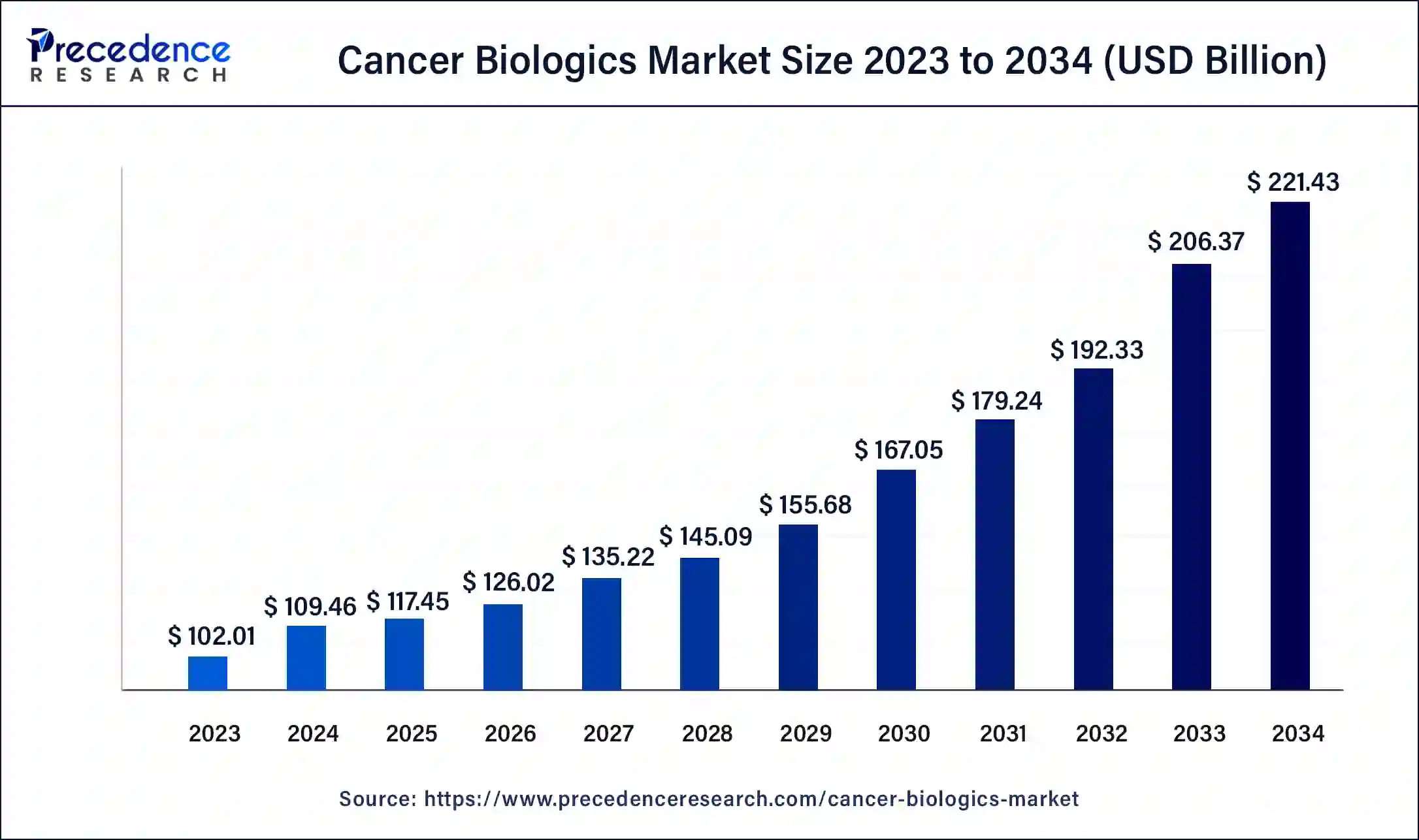
The global cancer biologics market size surpassed USD 102.01 billion in 2023 and is anticipated to attain around USD 214.59 billion by 2033, growing at a CAGR of 7.12% from 2024 to 2033.
Key Takeaways
- North America dominated the cancer biologics market in 2023
- Asia Pacific is expected to grow steadily in the market during the forecast period.
- The monoclonal antibodies segment dominated the market by drug class in 2023.
- The cancer growth inhibitors segment is expected to grow to the highest CAGR in the market by application during the forecast period.
- The blood cancer segment dominated the market by application in 2023.
- The lung cancer segment is expected to grow to the highest CAGR in the market by application during the forecast period.
- In 2023, the hospital segment dominated the market by end-use.
- The cancer center segment is expected to grow to the highest CAGR in the market by end-use during the forecast period.
The global cancer biologics market has witnessed substantial growth in recent years, driven by increasing incidences of cancer worldwide and advancements in biotechnology. Cancer biologics, which include monoclonal antibodies, cancer vaccines, and cytokine therapies, offer targeted treatment options with fewer side effects compared to traditional chemotherapy. These biologics are designed to specifically target cancer cells, thereby improving efficacy and reducing harm to healthy cells. The market has seen significant investments in research and development, leading to the introduction of innovative biologic therapies for various types of cancer.
Get a Sample: https://www.precedenceresearch.com/sample/4005
Growth Factors:
Several factors contribute to the growth of the cancer biologics market. Firstly, the rising prevalence of cancer globally, attributed to factors such as aging populations, lifestyle changes, and environmental factors, has increased the demand for effective treatment options. Additionally, the growing understanding of the molecular mechanisms underlying cancer has led to the development of targeted biologic therapies tailored to specific cancer types and genetic profiles. Moreover, advancements in biotechnology, including genetic engineering and protein engineering techniques, have facilitated the development of novel biologics with enhanced efficacy and reduced toxicity.
Region Insights:
The cancer biologics market exhibits regional variations driven by factors such as healthcare infrastructure, regulatory policies, and prevalence of specific cancer types. North America dominates the market, attributed to the presence of key biopharmaceutical companies, well-established healthcare systems, and high adoption rates of biologic therapies. Europe follows closely, with significant investments in cancer research and development and favorable reimbursement policies. The Asia-Pacific region is expected to witness rapid growth, fueled by increasing healthcare expenditure, rising awareness about cancer treatment options, and expanding access to advanced biologic therapies.
Cancer Biologics Market Dynamics
Drivers:
Several drivers propel the growth of the cancer biologics market. One key driver is the increasing demand for targeted therapies with higher efficacy and fewer side effects compared to conventional chemotherapy. Additionally, the growing trend towards personalized medicine, driven by advancements in genomics and molecular diagnostics, has spurred the development of biologic therapies tailored to individual patients' genetic profiles. Furthermore, strategic collaborations and partnerships between biopharmaceutical companies and research institutions have accelerated the pace of innovation and product development in the cancer biologics market.
Opportunities:
The cancer biologics market presents numerous opportunities for growth and innovation. One significant opportunity lies in expanding the application of biologic therapies beyond traditional cancer types to rare and difficult-to-treat cancers. Additionally, the development of combination therapies, involving the use of biologics in conjunction with other treatment modalities such as chemotherapy, radiation therapy, and immunotherapy, holds promise for improved treatment outcomes. Moreover, the emergence of biosimilars, which are highly similar versions of approved biologic therapies, presents opportunities for cost savings and increased access to cancer treatment.
Challenges:
Despite the promising growth prospects, the cancer biologics market faces several challenges. One major challenge is the high cost of biologic therapies, which limits access for patients in low- and middle-income countries and strains healthcare budgets in developed countries. Moreover, the complex manufacturing processes involved in producing biologic drugs pose challenges in terms of scalability, cost-effectiveness, and ensuring product quality and consistency. Additionally, regulatory hurdles, including stringent approval requirements and patent protections, can impede market entry for new biologic therapies and biosimilars. Furthermore, the emergence of resistance to biologic therapies and the potential for adverse effects underscore the need for ongoing research and development efforts to improve treatment outcomes and patient safety.
Recent Developments
- Dr. Reddy's Laboratories declared in March 2024 that Versavo® (bevacizumab) would be available in the UK. Avastin's biosimilar Versavo is effective against multiple cancer types, such as advanced non-squamous non-small cell lung cancer, metastatic colorectal cancer, ovarian cancer, recurrent glioblastoma, metastatic renal cell carcinoma, advanced cervical cancer, and metastatic breast cancer.
- BioNTech and Duality Biologics declared in January 2024 that a Phase III trial of a medication for breast cancer has begun. BioNTech and Duality are conducting a Phase III trial to evaluate their antibody-drug conjugate in patients whose tumors have low levels of progesterone or estrogen and respond to these hormones. This corresponds to between 40% and 45% of patients with metastatic breast cancer who are on Herceptin for HER2.
- Angle, a liquid biopsy firm that provides diagnostic solutions for circulating tumor cells (CTCs) in drug development, research, and clinical oncology, announced in November 2023 that the Portrait PD-L1 test would be available for CTCs to assess PD-L1 protein expression.
- In April 2023, TORL BioTherapeutics LLC, a biopharmaceutical company focused on developing new biologics for cancer treatment, announced its public launch and the closing of a $158 million Series B financing.
Cancer Biologics Market Companies
- Abbott
- Angel
- Amgen, Inc
- AstraZeneca
- BioNTech
- Bristol-Mayer Squibb Company
- Dr. Reddy's Laboratories
- Duality Biologics
- Eli Lilly and Company
- F.Hoffmann-La Roche Ltd.
- Gilead Sciences, Inc.
- Glenmark Pharmaceuticals Ltd
- GSK plc.
- Ichnos Sciences Inc
- Johnson & Johnson Services, Inc
- Pfizer, Inc
- TFC Therapeutics
Segments Covered in the Report
By Drug Class
- Monoclonal Antibodies
- Naked Monoclonal Antibodies
- Conjugated Monoclonal Antibodies
- Bispecific Monoclonal Antibodies
- Cancer Growth Inhibitors
- Tyrosine Kinase Inhibitor
- Mtor Inhibitors
- Proteasome Inhibitors
- Others
- Vaccines
- Preventive Vaccines
- Therapeutic Vaccines
- Recombinants Proteins
- CAR-T Cells
- Angiogenesis Inhibitors
- Interleukins (IL)
- Interferons (IFN)
- Gene Therapy
- Others
By Applications
- Blood Cancer
- Lung Cancer
- Breast Cancer
- Colorectal Cancer
- Prostate Cancer
- Gastric Cancer
- Ovarian Cancer
- Skin Cancer
- Liver Cancer
- Others
By End use
- Hospitals
- Cancer Center
- Academics & Research Institutes
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/


0 Comments