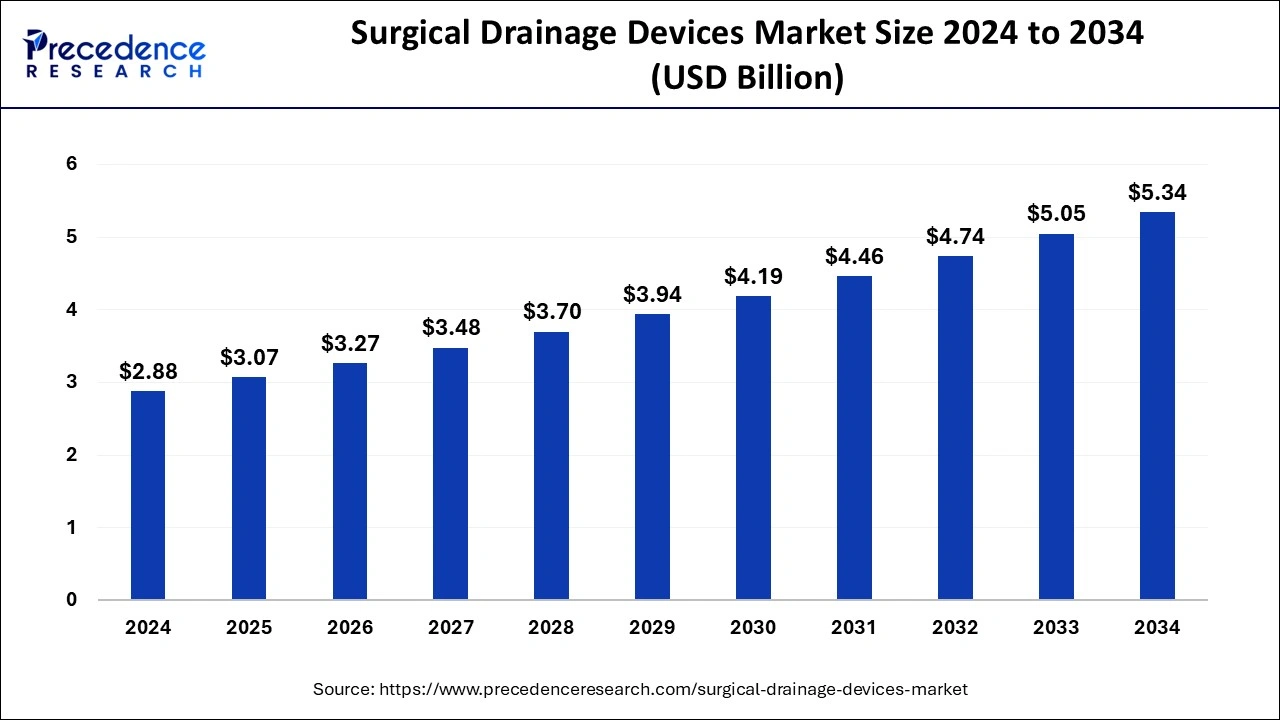
The global surgical drainage devices market size reached USD 2.71 billion in 2023 and is anticipated to hit around USD 5.05 billion by 2033, growing at a CAGR of 6.42% from 2024 to 2033.
Key Points
- North America has contributed more than 41% of the market share in 2023.
- Asia-Pacific is estimated to be the fastest-growing during the forecast period of 2024-2033.
- By product type, the active drains segment has held a major market share of 81% in 2023.
- By product type, the passive drains segment is the fastest-growing during the forecast period.
- By application, the thoracic and cardiovascular surgeries segment has accounted for more than 25% of the market share in 2023.
- By application, the neurosurgical procedures segment is the fastest-growing during the forecast period.
- By end-user, the hospitals segment has generated more than 46% of the market share in 2023.
- Based on the end-user, the ambulatory surgical center segment is the fastest-growing during the forecast period.
The surgical drainage devices market plays a critical role in post-operative care by facilitating the removal of excess fluids and preventing complications such as infections and hematomas. These devices are essential for promoting healing and reducing the risk of complications following surgical procedures. The market encompasses a wide range of products, including closed suction drainage systems, open drainage systems, and active drainage systems, which are used across various surgical specialties.
Get a Sample: https://www.precedenceresearch.com/sample/3983
Growth Factors:
Several factors contribute to the growth of the surgical drainage devices market. The increasing prevalence of chronic diseases and the rising demand for surgical interventions drive the need for effective drainage solutions. Technological advancements in drainage device design and materials improve efficacy, safety, and patient comfort, thereby expanding the market. Moreover, the growing adoption of minimally invasive surgical techniques and the rising geriatric population, prone to surgical interventions, further stimulate market growth.
Regional Insights
The global surgical drainage devices market exhibits regional variations in demand and adoption. North America and Europe dominate the market, driven by advanced healthcare infrastructure, high surgical procedure volumes, and favorable reimbursement policies. The Asia-Pacific region, on the other hand, represents a rapidly growing market fueled by increasing healthcare expenditure, rising awareness about surgical interventions, and expanding access to healthcare services. Emerging economies in Asia-Pacific, such as China, India, and Southeast Asian countries, offer significant growth opportunities due to improving healthcare infrastructure and a growing patient population.
Surgical Drainage Devices Market Dynamics
Drivers
Several drivers propel the growth of the surgical drainage devices market. The increasing number of surgical procedures, driven by demographic trends such as population growth and aging, contributes to market expansion. Additionally, advancements in surgical techniques and perioperative care enhance the demand for innovative drainage solutions. Furthermore, the growing prevalence of lifestyle-related diseases and the rising burden of chronic conditions necessitate surgical interventions, driving market growth.
Opportunities
The surgical drainage devices market presents opportunities for innovation and expansion. The integration of advanced technologies, such as vacuum-assisted closure (VAC) systems and negative pressure wound therapy (NPWT), enhances the effectiveness of drainage devices and improves patient outcomes. Moreover, the rising demand for disposable and single-use drainage devices due to infection control concerns and cost-effectiveness creates opportunities for market players to diversify their product offerings and cater to evolving customer needs.
Challenges
Despite its growth prospects, the surgical drainage devices market faces challenges such as pricing pressures, reimbursement uncertainties, and regulatory hurdles. The competitive landscape is characterized by intense competition among market players, leading to pricing pressures and margin erosion. Additionally, regulatory requirements for product approval and compliance pose challenges for market entry and product commercialization. Moreover, the economic impact of the COVID-19 pandemic has disrupted healthcare systems worldwide, affecting surgical procedure volumes and market dynamics.
Recent Developments
- In October 2023, Haermonics, a MedTech company, launched Haermonics Pure, a medical device. The device is capable of self-flushing the chest cavity of patients who have undergone heart surgery. The device can prevent the second surgery that is needed for the drainage of pus residual blood and blood clots and prevent life-threatening complications.
- In February 2023, Fujifilm launched an advanced endoscopic ultrasound machine. The machine will be able to provide enhanced diagnostic precision and image clarity. These features are useful in the management of Gallbladder drainage and bile duct drainage.
Surgical Drainage Devices Market Companies
- Acelity
- Cardinal Health
- Cook Medical
- Conva Tech Inc.
- C. R. Bard, Inc.
- Ethicon USA, LLC
- Medtronic
- Medline Industries Inc.
- Stryker
- Teleflex Incorporated
- Zimmer Biomet
Segments Covered in the Report
By Product Types
- Active Drains
- Jackson-Pratt Drain
- Hemovac Drain
- Blake Drain
- Negative Pressure Wound Therapy
- Redivac Drain
- EVD & Lumbar Drain
- Chest Tube
- Others
- Passive Drains
- Penrose Drain
- Others
By Applications
- Thoracic and Cardiovascular Surgeries
- Neurosurgical Procedures
- Abdominal Surgery
- Orthopedics
- Others
By End-users
- Hospitals
- Ambulatory Surgical Centers
- Clinics
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/


0 Comments