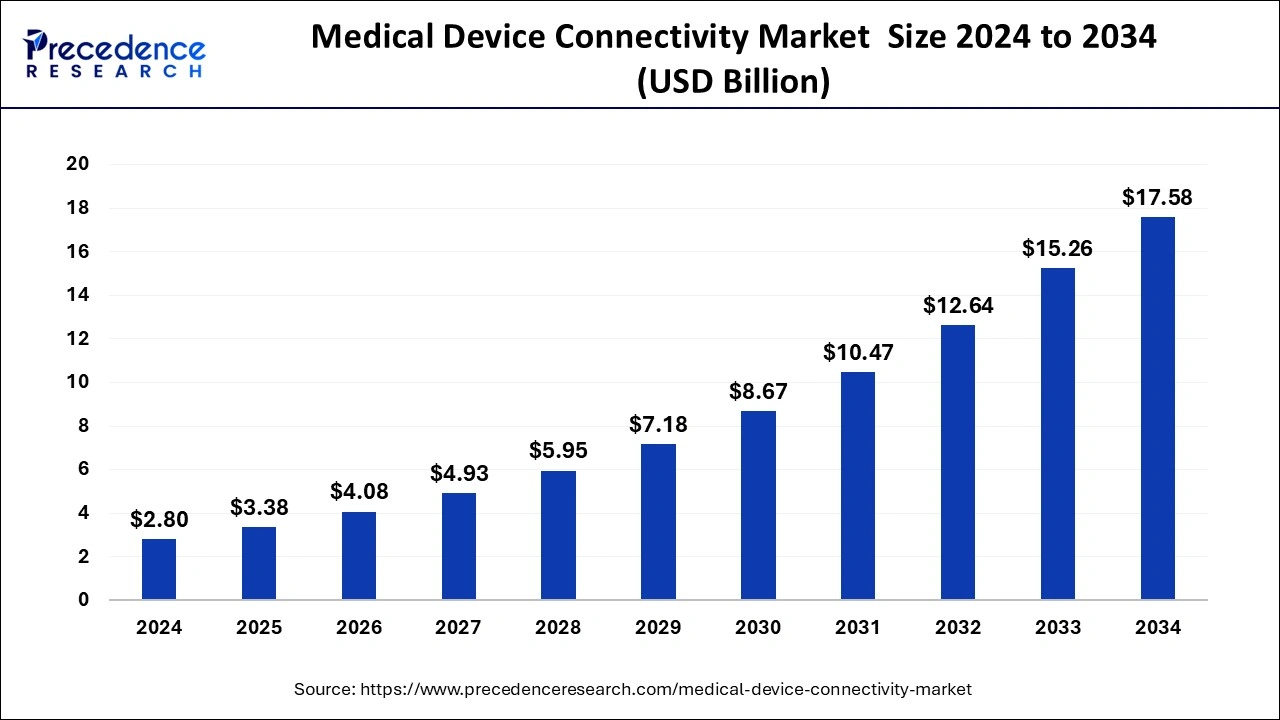
The global medical device connectivity market size reached USD 2.32 billion in 2023 and is anticipated to hit around USD 15.26 billion by 2033, growing at a CAGR of 20.74% from 2024 to 2033.
Key Points
- North America has contributed 36% of market share in 2023.
- Asia Pacific is estimated to expand the fastest CAGR of 25.7% between 2024 and 2033.
- By components, in 2023, the wireless segment held the highest market share of 48%.
- By components, the wired segment is anticipated to witness rapid growth at a significant CAGR during the projected period.
- By application, the hospitals segment has held the biggest market share in 2023.
- By application, the ambulatory care centers segment is anticipated to witness significant growth over the projected period.
The Medical Device Connectivity Market has witnessed substantial growth in recent years, driven by the increasing adoption of electronic health records (EHR) and the demand for real-time patient data monitoring. Medical device connectivity refers to the integration of medical devices with information systems to enable data exchange and communication between devices, healthcare providers, and patients. This connectivity facilitates the seamless transfer of patient data, enhances clinical workflows, and improves the quality of patient care. With advancements in technology, such as the Internet of Things (IoT) and wireless communication, medical device connectivity solutions have become more sophisticated and widely adopted across healthcare settings globally.
Get a Sample: https://www.precedenceresearch.com/sample/3996
Growth Factors:
Several factors contribute to the growth of the medical device connectivity market. One key driver is the growing emphasis on healthcare digitization and the adoption of electronic medical records (EMR) systems. Healthcare organizations are increasingly investing in connectivity solutions to streamline data management processes, reduce errors, and improve operational efficiency. Additionally, the rising prevalence of chronic diseases and the need for continuous patient monitoring have fueled the demand for connected medical devices. These devices enable remote monitoring of patients' vital signs, medication adherence, and disease management, leading to better outcomes and reduced healthcare costs.
Furthermore, regulatory mandates and initiatives promoting interoperability and data exchange in healthcare have stimulated market growth. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have issued guidelines and standards to ensure the safe and effective implementation of medical device connectivity solutions. Compliance with these standards is driving the development of interoperable and secure connectivity solutions, fostering market expansion.
Region Insights:
The medical device connectivity market exhibits significant regional variation, influenced by factors such as healthcare infrastructure, technological advancements, and regulatory environment. North America dominates the market, driven by the presence of a well-established healthcare IT sector, high adoption of electronic health records, and supportive government initiatives. The region is characterized by extensive investments in healthcare IT infrastructure and a strong focus on interoperability and data exchange standards.
Europe is another lucrative market for medical device connectivity, propelled by increasing healthcare expenditure, rising demand for advanced healthcare solutions, and favorable government policies promoting digitization. Countries such as Germany, the UK, and France are at the forefront of adoption, with a growing number of healthcare facilities implementing connected medical devices to enhance patient care and operational efficiency.
In the Asia-Pacific region, rapid economic development, expanding healthcare infrastructure, and growing awareness about healthcare IT solutions are driving market growth. Countries like China, India, and Japan are witnessing increased adoption of medical device connectivity solutions, supported by government initiatives to modernize healthcare systems and improve access to quality care in rural areas.
Medical Device Connectivity Market Dynamics
Drivers:
Several drivers contribute to the growth of the medical device connectivity market. One of the primary drivers is the increasing demand for real-time patient monitoring and remote healthcare services. Connected medical devices enable healthcare providers to monitor patients' health status remotely, facilitate early intervention, and reduce hospital readmissions. This is particularly beneficial for managing chronic diseases, elderly care, and post-operative monitoring, where continuous monitoring plays a crucial role in improving patient outcomes and reducing healthcare costs.
Moreover, the growing emphasis on value-based care and population health management is driving the adoption of medical device connectivity solutions. Healthcare organizations are increasingly focusing on preventive care, care coordination, and patient engagement to improve overall health outcomes and reduce the burden on healthcare systems. Connected medical devices play a vital role in supporting these initiatives by providing actionable insights, facilitating care coordination among providers, and empowering patients to actively participate in their healthcare management.
Opportunities:
The medical device connectivity market presents numerous opportunities for stakeholders across the healthcare ecosystem. One significant opportunity lies in the integration of artificial intelligence (AI) and machine learning (ML) technologies with medical device connectivity solutions. AI-powered analytics can leverage the vast amounts of data generated by connected medical devices to derive actionable insights, predict health outcomes, and personalize treatment plans. By harnessing AI and ML capabilities, healthcare providers can unlock new opportunities for precision medicine, predictive analytics, and population health management, leading to improved clinical decision-making and patient outcomes.
Another opportunity lies in the expansion of telehealth and remote patient monitoring services. The COVID-19 pandemic has accelerated the adoption of telehealth solutions, driving demand for connected medical devices that enable remote monitoring of patients' vital signs, chronic conditions, and post-operative recovery. As healthcare delivery models continue to evolve towards remote care and virtual consultations, there is a growing need for interoperable and user-friendly medical device connectivity solutions that support seamless data exchange between patients, providers, and caregivers.
Restraints:
Despite the promising growth prospects, the medical device connectivity market faces certain challenges and restraints. One of the primary challenges is the complexity of interoperability and data exchange standards. With a wide variety of medical devices from different manufacturers and varying communication protocols, achieving seamless interoperability and data integration remains a significant challenge for healthcare organizations. Lack of standardized protocols and interoperability barriers can hinder the widespread adoption of medical device connectivity solutions and limit their effectiveness in improving patient care and clinical workflows.
Moreover, concerns regarding data privacy, security, and regulatory compliance pose significant challenges to market growth. Connected medical devices collect and transmit sensitive patient health data, raising concerns about data breaches, unauthorized access, and compliance with regulations such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe. Healthcare organizations must implement robust security measures, encryption protocols, and access controls to safeguard patient data and ensure compliance with regulatory requirements. Failure to address these security concerns can undermine trust in medical device connectivity solutions and impede market growth.
Recent Developments
- In October 2023, Philips unveiled new interoperability features aimed at providing hospitals with a comprehensive patient health overview for enhanced monitoring and care coordination. By integrating Philips Capsule Medical Device Information Platform (MDIP) with Philips Patient Information Center iX (PIC iX), the company offers a unique patient monitoring ecosystem, bringing together diverse medical devices and systems onto a single interface. This interoperability allows clinicians to access streaming data from various device manufacturers on an open, secure platform, offering a new clinical perspective.
- In February 2023, the province of Nova Scotia collaborated with Nova Scotia Health Authority (NSHA) and IWK Health (IWK) to implement an integrated electronic care record system across the province, facilitated by Oracle Cerner. This technology aims to improve healthcare professionals' access to patient information.
- In 2021, Koninklijke Philips N.V. entered into an agreement to acquire Capsule Technologies Inc., a leading provider of medical device integration and data technologies to healthcare organizations. This strategic move was intended to bolster Philips' position in delivering connectivity solutions for patient care management within hospital settings.
- In 2021, Masimo Corporation introduced iSirona, a connectivity solution designed to integrate with electronic medical records (EMRs), surveillance monitoring, alarm management, mobile notifications, smart displays, and analytics.
Medical Device Connectivity Market Companies
- Cerner Corporation (U.S.)
- Medtronic (Ireland)
- Masimo (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- General Electric (U.S.)
- Stryker (U.S.)
- iHealth Labs Inc., (U.S.)
- Cisco Systems, Inc. (U.S.)
- Lantronix Inc. (U.S.)
- Infosys Limited (India)
- Silicon & Software Systems Ltd. (Ireland)
- Hill-Rom Services Inc. (U.S.)
- Silex Technology America, Inc (Japan)
- Digi International Inc. (U.S.)
- Baxter (U.S.)
- TE Connectivity (Switzerland)
- Bridge-Tech (U.S)
- MediCollector (U.S.)
Segments Covered in the Report
By Components
- Wireless
- Wired
- Hybrid Technologies
By Application
- Hospitals
- Home Healthcare Centers
- Diagnostic Centers
- Ambulatory Care Centers
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/


0 Comments