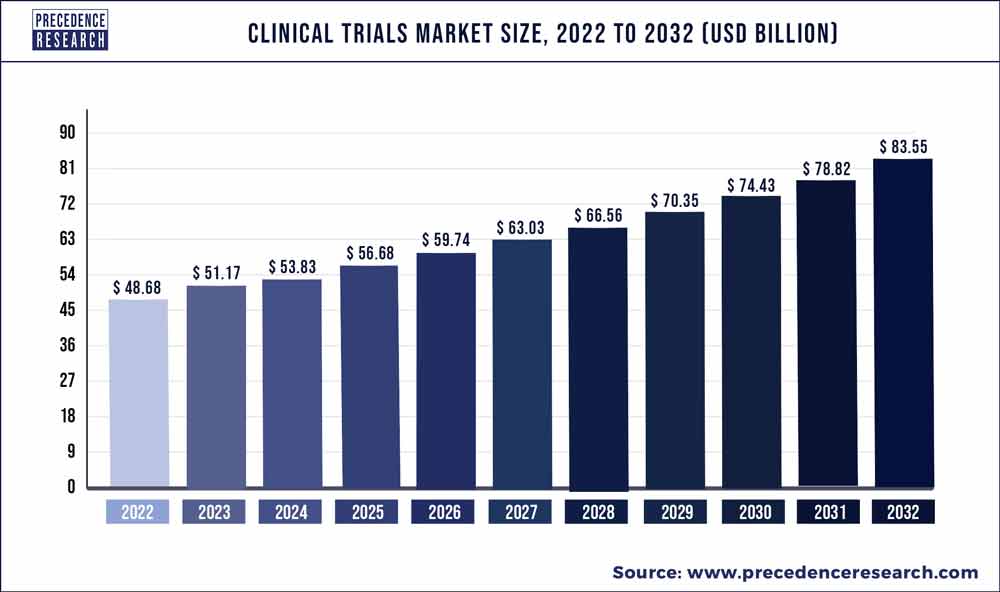
The global clinical trials market size reached USD 48.68 billion in 2022 and is predicted to hit USD 83.55 bn by 2032 with a representing CAGR of 5.6% from 2023 to 2032.
Key Takeaway
- North America has held 51.7% of the total market share in 2022.
- Asia Pacific region is growing at a CAGR of 6.8% during the forecast period.
- By indication, the oncology segment has held a market share of around 24.7% in 2022.
- The cardiovascular condition segment is growing at a CAGR of 6.4% during the forecast period.
- By sponsor, The pharmaceutical & biopharmaceutical companies has generated the largest revenue share of around 72% in 2022.
- By Indication by study design, the interventional trials market for autoimmune/inflammation has captured 83% market share in 2022.
- By study design, the interventional design segment has accounted revenue share of 46% in 2022.
- The expanded access trials segment is projected to register at a CAGR of 6.4% over the forecast period.
Clinical Trials are a type of clinical research that are performed in people and are intended at assessing a behavioral, surgical, or medical intervention. Clinical Trials act as the prime way employed by the scientists to investigate if a new therapy, a medical device or a novel drug is secure and efficient in individuals. A clinical trial is also regularly used to study if a new therapy is more competent and presents less detrimental side effects than the conventional treatments. In order to get the approval of U.S. FDA for the initiation of clinical trials, the scientists first have to perform laboratory tests and examinations in animals in order to test a probable therapy’s efficacy and safety. If these research studies display favorable outcomes, the U.S. FDA grants approval for the therapy to be assessed in humans. After the necessary approvals, Individuals can volunteer to participate in clinical trials in order to test medicinal interventions that include drugs, devices, biological products, radiological procedures, surgical procedures, and preventive care. Clinical trials comprise of five phases.
The phase 0 trials are the initial clinical trials performed among humans. These trials aim to study how a medication is handled in the human body and in what way it influences the body. The objective of phase I trials is to identify the best dosage of a new medication with minimal side effects. Phase II clinical trials further evaluate safety along with the working of a medication. Phase III trials corelate a new medication to the standard-of-care medication. Phase IV trials examine new drugs permitted by the FDA. The medication is evaluated in several thousands of patients.The global clinical trials market size was valued at USD 48.68 billion in 2022 and is predicted to hit USD 83.55 bn by 2032 with a registered CAGR of 5.6% during the forecast period 2023 to 2032.
Growth Factors:
Factors such as growing prevalence of chronic disorders, increasing number of clinical trials in developing regions, growing number of biologics, increasing demand for advanced treatments such as personalized medicines, outbreak of viral diseases, increasing cases of cancer globally, growing geriatric population and growing research and development expenditure are propelling the clinical trials market expansion across the globe. Additional aspects that are anticipated to fuel this industry are increasing popularity of contract research organizations (CROs) and technological innovations. Constant collaborations and partnerships between major market players in order to launch new and innovative technologies have enhanced the demand for overall market for the clinical trials market.
Contract research organizations (CROs) are vital to the biotech, pharmaceutical, and medical devices industries as they support efforts of the client company to test, enhance, and market the modern pharmaceutical products and medical devices.
Report Highlights:
- Among the phase segment, phase 3 dominated the overall market in 2020. Involvement of large number of participants and high cost is the major reason for high market share of phase 3 clinical trials.
- The interventional study accounted for the largest revenue in the study design segment with more than 46% share in 2020. Factors such as high use in drug and biologics studies and technological innovations drive the growth of interventional study.
- Oncology accounted for the largest revenue in the indication segment. High prevalence of cancer in developing regions is the key reason for high market share of oncology.
- The key companies included in the analysis are Parexel, IQVIA, Charles River Laboratory, Omnicare, Kendle, Chiltern, and Pharmaceutical Product Development, LLC. Among others. IQVIA accounted for a significant share of the global clinical trials market.
Key Market Players and Strategies:
The major companies operating in the worldwide clinical trials market are Parexel, IQVIA, Charles River Laboratory, Omnicare, Kendle, Chiltern, and Pharmaceutical Product Development, LLC. among others.
High investment in the research and development along with acquisition, mergers, and collaborations are the key strategies undertaken by companies operating in the global clinical trials market. Clinical trials are fundamental to the development of new treatment and vaccinations methods. The outbreak of SARS CoV-2 prompted many companies to undertake clinical trials in order to develop vaccines as well treatments against the viral disease. Ampio Pharmaceuticals, for example, undertook global clinical trials for the development of intravenous ("IV") treatment of novel corona virus disease (COVID-19).
Regional Analysis:
The report covers data for North America, Europe, Asia Pacific, Latin America, and Middle East and Africa. In 2020, North America dominated the global market with a market share of more than 52%. U.S. represented the highest share in the North American region primarily due to presence of latest healthcare infrastructure and early adoption of new technologies in clinical research. Furthermore, high investment by major market players also contributed to the high market share of the United States.
Europe was the second important market chiefly due to presence of skilled researchers and increasing research and development activities. High incidence of chronic disorders in the European region is also expected to boost the demand for clinical trials market in the near future. Asia Pacific is anticipated to grow at the maximum CAGR of around 6.7% in the forecast period due to high incidence of viral disorders. Latin America and the African and Middle Eastern region will display noticeable growth.
Market Segmentation
By Phase
- Phase 1
- Phase 2
- Phase 3
- Phase 4
By Study Design
- Observational
- Interventional
- Expanded Access
By Indication
- Oncology
- Autoimmune/Inflammation
- Diabetes
- Central Nervous System
- Cardiovascular
- Pain Management
- Others
By Geography
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa (MEA)
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/


0 Comments