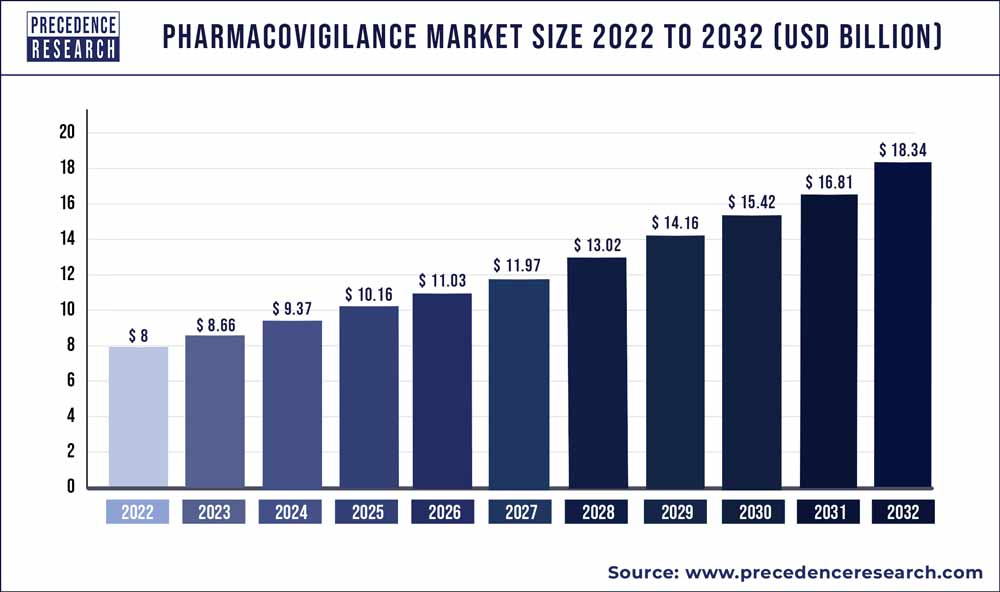
The pharmacovigilance market size is poised to grow by $ 16.23 billion by 2030 from $ 9.07 Billion in 2022, exhibiting a CAGR of 7.5% during the forecast period 2023-2032.
Key Takeaway:
- North America makrt has generated 31% of total revenue share in 2022.
- Asia Pacific region is expanding at a CAGR of 10.9% between 2023 to 2032.
- By end user, the pharmaceutical companies dominate the market with largest revenue share 42.8% in 2022.
- By service provider, the contract outsourcing segment has generated revenue share of 60% in 2022.
- By clinical trial phase, the phase IV segment has garnered revenue share of 76% in 2022.
- By process flow, signal detection has held revenue share of around 39.6% in 2022.
- By therapeutic area, oncology segment has captured revenue share of over 26.8% in 2022 with a CAGR of 11.2%.
- By type, spontaneous reporting has accounted revenue share of 30.4% in 2022.
The main objective of this report is to define, describe, and forecast the global market by types, application, manufacturers, and regions. The report provides detailed information about the major factors (drivers, restraints, opportunities, and industry-specific challenges) influencing the growth of the market. The report aims to strategically analyze the micro markets with respect to individual growth trends, prospects, and contributions to the global market. The report also attempts to forecast the market size of the 5 main regions: North America, Europe, Asia Pacific (APAC), Middle East and Africa (MEA), and Latin America. It strategically profiles the key market players and comprehensively analyzes their core competencies. It also tracks and analyzes competitive developments, such as joint ventures, mergers and acquisitions, new developments, and Research and Development (R&D) activities, in market.
Get a Sample: https://www.precedenceresearch.com/sample/1442
COVID-19 Impact
Since the COVID-19 virus outbreak in December 2019, the disease has spread to almost 100 countries around the globe with the World Health Organization declaring it a public health emergency. The global impacts of the coronavirus disease 2019 (COVID-19) are already starting to be felt, and will significantly affect the electric vehicle fuel cell market in 2020.
COVID-19 can affect the global economy in three main ways: by directly affecting production and demand, by creating supply chain and market disruption, and by its financial impact on firms and financial markets.
The outbreak of COVID-19 has brought effects on many aspects, like flight cancellations; travel bans and quarantines; restaurants closed; all indoor events restricted; over forty countries state of emergency declared; massive slowing of the supply chain; stock market volatility; falling business confidence, growing panic among the population, and uncertainty about future.
Major companies operating in this are
- ICON Plc
- Pharmaceutical Product Development LLC
- Parexel International Corporation
- IQVIA
- Quanticate
- Bioclinica
- Covance Inc.
- Accenture Plc
- IBM Corporation
- Novartis
Market Segmentation
By Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Service Provider
- In-house
- Contract Outsourcing
By End User
- Hospitals
- Pharmaceutical Companies
- Others
By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
By Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
By Process Flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Major highlights of Report:
- Figures related to sales volume, market remuneration, and segmental shares
- Featuring market dynamics
- Growth prospects and expansion graph
- PROCON study of direct & indirect sales channels
- Profiles of prominent traders, dealer, and distributors in the industry
Geographical landscape: North America, Europe, Asia-Pacific, South America, Middle East and Africa
- Industry forecasts based on region and at country level
- Data about sales volume recorded, industry share held, and profit margins amassed
- Overall remuneration and estimated growth rate for each regional market
Major Key Points Covered in Report:
Executive Summary: It includes key trends of the electric vehicle fuel cell market related to products, applications, and other crucial factors. It also provides analysis of the competitive landscape and CAGR and market size of the electric vehicle fuel cell market based on production and revenue.
Production and Consumption by Region: It covers all regional markets to which the research study relates. Prices and key players in addition to production and consumption in each regional market are discussed.
Key Players: Here, the report throws light on financial ratios, pricing structure, production cost, gross profit, sales volume, revenue, and gross margin of leading and prominent companies competing in the Electric vehicle fuel cell market.
Market Segments: This part of the report discusses product, application and other segments of the electric vehicle fuel cell market based on market share, CAGR, market size, and various other factors.
Research Methodology: This section discusses the research methodology and approach used to prepare the report. It covers data triangulation, market breakdown, market size estimation, and research design and/or programs.
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Process Flow Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Pharmacovigilance Market
5.1. COVID-19 Landscape: Pharmacovigilance Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Pharmacovigilance Market, By Clinical Trial Phase
8.1. Pharmacovigilance Market, by Clinical Trial Phase Type, 2021-2030
8.1.1. Preclinical Phase I
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Phase II
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Phase III
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Phase IV
8.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Pharmacovigilance Market, By Service Provider Type
9.1. Pharmacovigilance Market, by Service Provider Type, 2021-2030
9.1.1. In-house
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Contract Outsourcing
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Pharmacovigilance Market, By End User
10.1. Pharmacovigilance Market, by End User Type, 2021-2030
10.1.1. Hospitals
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Pharmaceutical Companies
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Others
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Pharmacovigilance Market, By Therapeutic Area
11.1. Pharmacovigilance Market, by Therapeutic Area Type, 2021-2030
11.1.1. Oncology
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Neurology
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Cardiology
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Respiratory Systems
11.1.4.1. Market Revenue and Forecast (2017-2030)
11.1.5. Others
11.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Pharmacovigilance Market, By Type
12.1. Pharmacovigilance Market, by Type, 2021-2030
12.1.1. Spontaneous Reporting
12.1.1.1. Market Revenue and Forecast (2017-2030)
12.1.2. Intensified ADR Reporting
12.1.2.1. Market Revenue and Forecast (2017-2030)
12.1.3. Targeted Spontaneous Reporting
12.1.3.1. Market Revenue and Forecast (2017-2030)
12.1.4. Cohort Event Monitoring
12.1.4.1. Market Revenue and Forecast (2017-2030)
12.1.5. EHR Mining
12.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 13. Global Pharmacovigilance Market, By Process Flow
13.1. Pharmacovigilance Market, by Service Provider Type, 2021-2030
13.1.1. Case Data Management
13.1.1.1. Market Revenue and Forecast (2017-2030)
13.1.2. Signal Detection
13.1.2.1. Market Revenue and Forecast (2017-2030)
13.1.3. Risk Management System
13.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 14. Global Pharmacovigilance Market, Regional Estimates and Trend Forecast
14.1. North America
14.1.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.1.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.1.3. Market Revenue and Forecast, by End User (2017-2030)
14.1.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.1.5. Market Revenue and Forecast, by Type (2017-2030)
14.1.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.1.7. U.S.
14.1.7.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.1.7.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.1.7.3. Market Revenue and Forecast, by End User (2017-2030)
14.1.7.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.1.8. Market Revenue and Forecast, by Type (2017-2030)
14.1.8.1. Market Revenue and Forecast, by Process Flow (2017-2030)
14.1.9. Rest of North America
14.1.9.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.1.9.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.1.9.3. Market Revenue and Forecast, by End User (2017-2030)
14.1.9.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.1.10. Market Revenue and Forecast, by Type (2017-2030)
14.1.11. Market Revenue and Forecast, by Process Flow (2017-2030)
14.1.11.1.
14.2. Europe
14.2.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.2.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.2.3. Market Revenue and Forecast, by End User (2017-2030)
14.2.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.2.5. Market Revenue and Forecast, by Type (2017-2030)
14.2.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.2.7.
14.2.8. UK
14.2.8.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.2.8.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.2.8.3. Market Revenue and Forecast, by End User (2017-2030)
14.2.9. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.2.10. Market Revenue and Forecast, by Type (2017-2030)
14.2.10.1. Market Revenue and Forecast, by Process Flow (2017-2030)
14.2.11. Germany
14.2.11.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.2.11.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.2.11.3. Market Revenue and Forecast, by End User (2017-2030)
14.2.12. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.2.13. Market Revenue and Forecast, by Type (2017-2030)
14.2.14. Market Revenue and Forecast, by Process Flow (2017-2030)
14.2.14.1.
14.2.15. France
14.2.15.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.2.15.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.2.15.3. Market Revenue and Forecast, by End User (2017-2030)
14.2.15.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.2.16. Market Revenue and Forecast, by Type (2017-2030)
14.2.16.1. Market Revenue and Forecast, by Process Flow (2017-2030)
14.2.17. Rest of Europe
14.2.17.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.2.17.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.2.17.3. Market Revenue and Forecast, by End User (2017-2030)
14.2.17.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.2.18. Market Revenue and Forecast, by Type (2017-2030)
14.2.18.1. Market Revenue and Forecast, by Process Flow (2017-2030)
14.3. APAC
14.3.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.3.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.3.3. Market Revenue and Forecast, by End User (2017-2030)
14.3.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.3.5. Market Revenue and Forecast, by Type (2017-2030)
14.3.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.3.7. India
14.3.7.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.3.7.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.3.7.3. Market Revenue and Forecast, by End User (2017-2030)
14.3.7.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.3.8. Market Revenue and Forecast, by Type (2017-2030)
14.3.9. Market Revenue and Forecast, by Process Flow (2017-2030)
14.3.10. China
14.3.10.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.3.10.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.3.10.3. Market Revenue and Forecast, by End User (2017-2030)
14.3.10.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.3.11. Market Revenue and Forecast, by Type (2017-2030)
14.3.11.1. Market Revenue and Forecast, by Process Flow (2017-2030)
14.3.12. Japan
14.3.12.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.3.12.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.3.12.3. Market Revenue and Forecast, by End User (2017-2030)
14.3.12.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.3.12.5. Market Revenue and Forecast, by Type (2017-2030)
14.3.12.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.3.13. Rest of APAC
14.3.13.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.3.13.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.3.13.3. Market Revenue and Forecast, by End User (2017-2030)
14.3.13.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.3.13.5. Market Revenue and Forecast, by Type (2017-2030)
14.3.13.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.4. MEA
14.4.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.4.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.4.3. Market Revenue and Forecast, by End User (2017-2030)
14.4.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.4.5. Market Revenue and Forecast, by Type (2017-2030)
14.4.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.4.7. GCC
14.4.7.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.4.7.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.4.7.3. Market Revenue and Forecast, by End User (2017-2030)
14.4.7.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.4.8. Market Revenue and Forecast, by Type (2017-2030)
14.4.9. Market Revenue and Forecast, by Process Flow (2017-2030)
14.4.10. North Africa
14.4.10.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.4.10.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.4.10.3. Market Revenue and Forecast, by End User (2017-2030)
14.4.10.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.4.11. Market Revenue and Forecast, by Type (2017-2030)
14.4.12. Market Revenue and Forecast, by Process Flow (2017-2030)
14.4.13. South Africa
14.4.13.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.4.13.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.4.13.3. Market Revenue and Forecast, by End User (2017-2030)
14.4.13.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.4.13.5. Market Revenue and Forecast, by Type (2017-2030)
14.4.13.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.4.14. Rest of MEA
14.4.14.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.4.14.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.4.14.3. Market Revenue and Forecast, by End User (2017-2030)
14.4.14.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.4.14.5. Market Revenue and Forecast, by Type (2017-2030)
14.4.14.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.5. Latin America
14.5.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.5.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.5.3. Market Revenue and Forecast, by End User (2017-2030)
14.5.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.5.5. Market Revenue and Forecast, by Type (2017-2030)
14.5.6. Market Revenue and Forecast, by Process Flow (2017-2030)
14.5.7. Brazil
14.5.7.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.5.7.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.5.7.3. Market Revenue and Forecast, by End User (2017-2030)
14.5.7.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.5.8. Market Revenue and Forecast, by Type (2017-2030)
14.5.8.1. Market Revenue and Forecast, by Process Flow (2017-2030)
14.5.9. Rest of LATAM
14.5.9.1. Market Revenue and Forecast, by Clinical Trial Phase (2017-2030)
14.5.9.2. Market Revenue and Forecast, by Service Provider (2017-2030)
14.5.9.3. Market Revenue and Forecast, by End User (2017-2030)
14.5.9.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
14.5.9.5. Market Revenue and Forecast, by Type (2017-2030)
14.5.9.6. Market Revenue and Forecast, by Process Flow (2017-2030)
Chapter 15. Company Profiles
15.1. ICON Plc
15.1.1. Company Overview
15.1.2. Product Offerings
15.1.3. Financial Performance
15.1.4. Recent Initiatives
15.2. Pharmaceutical Product Development LLC
15.2.1. Company Overview
15.2.2. Product Offerings
15.2.3. Financial Performance
15.2.4. Recent Initiatives
15.3. Parexel International Corporation
15.3.1. Company Overview
15.3.2. Product Offerings
15.3.3. Financial Performance
15.3.4. Recent Initiatives
15.4. IQVIA
15.4.1. Company Overview
15.4.2. Product Offerings
15.4.3. Financial Performance
15.4.4. Recent Initiatives
15.5. Quanticate
15.5.1. Company Overview
15.5.2. Product Offerings
15.5.3. Financial Performance
15.5.4. Recent Initiatives
15.6. Bioclinica
15.6.1. Company Overview
15.6.2. Product Offerings
15.6.3. Financial Performance
15.6.4. Recent Initiatives
15.7. Covance Inc.
15.7.1. Company Overview
15.7.2. Product Offerings
15.7.3. Financial Performance
15.7.4. Recent Initiatives
15.8. Accenture Plc
15.8.1. Company Overview
15.8.2. Product Offerings
15.8.3. Financial Performance
15.8.4. Recent Initiatives
15.9. IBM Corporation
15.9.1. Company Overview
15.9.2. Product Offerings
15.9.3. Financial Performance
15.9.4. Recent Initiatives
15.10. Novartis
15.10.1. Company Overview
15.10.2. Product Offerings
15.10.3. Financial Performance
15.10.4. Recent Initiatives
Chapter 16. Research Methodology
16.1. Primary Research
16.2. Secondary Research
16.3. Assumptions
Chapter 17. Appendix
17.1. About Us
17.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.pharma-geek.com


0 Comments