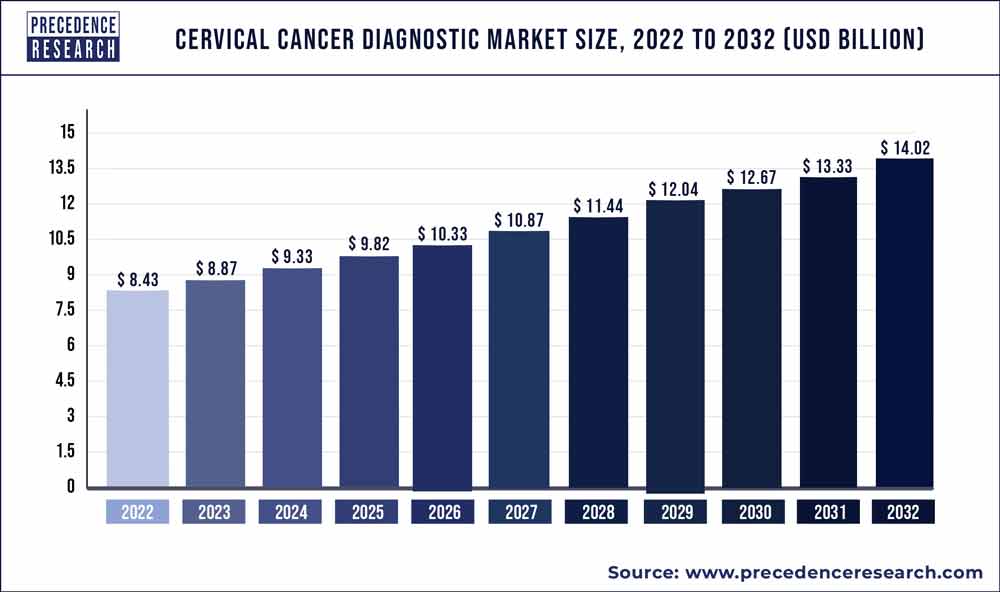
According to the research report, the global cervical cancer diagnostic market size is expected to touch USD 14.02 Billion by 2032, from USD 8.43 Billion in 2022, growing with a significant CAGR of 5.22% from 2023 to 2032.
The cervical cancer diagnostic report offers a comprehensive study of the current state expected at the major drivers, market strategies, and key vendors’ growth. The report presents energetic visions to conclude and study the market size, market hopes, and competitive surroundings. The research also focuses on the important achievements of the market, research & development, and regional growth of the leading competitors operating in the market. The current trends of the global cervical cancer diagnostic in conjunction with the geographical landscape of this vertical have also been included in this report.
The report offers intricate dynamics about different aspects of the global cervical cancer diagnostic market, which aids companies operating in the market in making strategic development decisions. The study also elaborates on significant changes that are highly anticipated to configure growth of the global cervical cancer diagnostic during the forecast period. It also includes a key indicator assessment that highlights growth prospects of this market and estimates statistics related to growth of the market in terms of value (US$ Mn) and volume (tons).
Sample Link @ https://www.precedenceresearch.com/sample/3178
This study covers a detailed segmentation of the global cervical cancer diagnostic market, along with key information and a competition outlook. The report mentions company profiles of players that are currently dominating the global cervical cancer diagnostic market, wherein various developments, expansions, and winning strategies practiced and implemented by leading players have been presented in detail.
Key Players
- Zilico
- Siemens Healthineers AG
- QIAGEN NV
- Abbott Laboratories
- Becton, Dickinson and Co.
- Quest Diagnostics Inc.
- F. Hoffmann-La Roche Ltd.
- Guided Therapeutics
- Hologic Inc.
- Bio-Rad Laboratories Inc.
Market Segmentation
By Diagnostic Test
- Pap Smear Test
- HPV Test
- Colposcopy
- Biopsy and Endocervical Curettage
- Other Diagnostic Tests
By End-user
- Hospitals
- Specialty Clinics
- Cancer and Radiation Therapy Centers
- Diagnostic Centers
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Research Methodology
The research methodology adopted by analysts for compiling the global cervical cancer diagnostic report is based on detailed primary as well as secondary research. With the help of in-depth insights of the market-affiliated information that is obtained and legitimated by market-admissible resources, analysts have offered riveting observations and authentic forecasts for the global market.
During the primary research phase, analysts interviewed market stakeholders, investors, brand managers, vice presidents, and sales and marketing managers. Based on data obtained through interviews of genuine resources, analysts have emphasized the changing scenario of the global market.
For secondary research, analysts scrutinized numerous annual report publications, white papers, market association publications, and company websites to obtain the necessary understanding of the global cervical cancer diagnostic market.
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology (Premium Insights)
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Cervical Cancer Diagnostic Market
5.1. COVID-19 Landscape: Cervical Cancer Diagnostic Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Cervical Cancer Diagnostic Market, By Diagnostic Test
8.1. Cervical Cancer Diagnostic Market, by Diagnostic Test, 2023-2032
8.1.1. Pap Smear Test
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. HPV Test
8.1.2.1. Market Revenue and Forecast (2020-2032)
8.1.3. Colposcopy
8.1.3.1. Market Revenue and Forecast (2020-2032)
8.1.4. Biopsy and Endocervical Curettage
8.1.4.1. Market Revenue and Forecast (2020-2032)
8.1.5. Other Diagnostic Tests
8.1.5.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global Cervical Cancer Diagnostic Market, By End-user
9.1. Cervical Cancer Diagnostic Market, by End-user, 2023-2032
9.1.1. Hospitals
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Specialty Clinics
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Cancer and Radiation Therapy Centers
9.1.3.1. Market Revenue and Forecast (2020-2032)
9.1.4. Diagnostic Centers
9.1.4.1. Market Revenue and Forecast (2020-2032)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global Cervical Cancer Diagnostic Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.1.2. Market Revenue and Forecast, by End-user (2020-2032)
10.1.3. U.S.
10.1.3.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.1.3.2. Market Revenue and Forecast, by End-user (2020-2032)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.1.4.2. Market Revenue and Forecast, by End-user (2020-2032)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.2.2. Market Revenue and Forecast, by End-user (2020-2032)
10.2.3. UK
10.2.3.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.2.3.2. Market Revenue and Forecast, by End-user (2020-2032)
10.2.4. Germany
10.2.4.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.2.4.2. Market Revenue and Forecast, by End-user (2020-2032)
10.2.5. France
10.2.5.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.2.5.2. Market Revenue and Forecast, by End-user (2020-2032)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.2.6.2. Market Revenue and Forecast, by End-user (2020-2032)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.3.2. Market Revenue and Forecast, by End-user (2020-2032)
10.3.3. India
10.3.3.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.3.3.2. Market Revenue and Forecast, by End-user (2020-2032)
10.3.4. China
10.3.4.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.3.4.2. Market Revenue and Forecast, by End-user (2020-2032)
10.3.5. Japan
10.3.5.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.3.5.2. Market Revenue and Forecast, by End-user (2020-2032)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.3.6.2. Market Revenue and Forecast, by End-user (2020-2032)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.4.2. Market Revenue and Forecast, by End-user (2020-2032)
10.4.3. GCC
10.4.3.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.4.3.2. Market Revenue and Forecast, by End-user (2020-2032)
10.4.4. North Africa
10.4.4.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.4.4.2. Market Revenue and Forecast, by End-user (2020-2032)
10.4.5. South Africa
10.4.5.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.4.5.2. Market Revenue and Forecast, by End-user (2020-2032)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.4.6.2. Market Revenue and Forecast, by End-user (2020-2032)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.5.2. Market Revenue and Forecast, by End-user (2020-2032)
10.5.3. Brazil
10.5.3.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.5.3.2. Market Revenue and Forecast, by End-user (2020-2032)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Forecast, by Diagnostic Test (2020-2032)
10.5.4.2. Market Revenue and Forecast, by End-user (2020-2032)
Chapter 11. Company Profiles
11.1. Zilico
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Siemens Healthineers AG
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. QIAGEN NV
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Abbott Laboratories
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. Becton, Dickinson and Co.
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Quest Diagnostics Inc.
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. F. Hoffmann-La Roche Ltd.
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Guided Therapeutics
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Hologic Inc.
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Bio-Rad Laboratories Inc.
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 774 402 6168
Email: sales@precedenceresearch.com
Website: https://www.precedenceresearch.com
Blog: https://www.pharma-geek.com


0 Comments