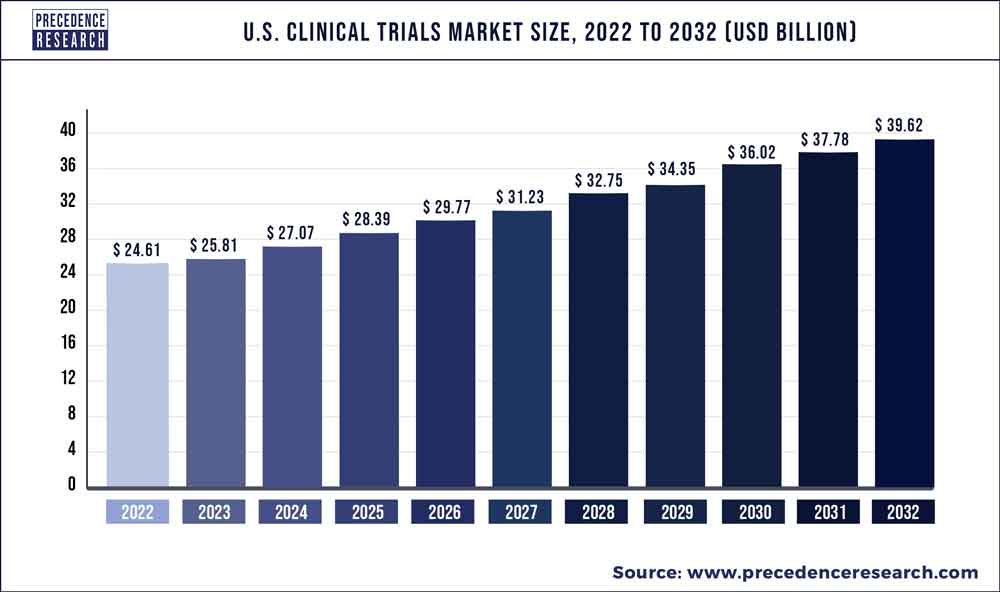
The US clinical trials market would grow at a CAGR of 4.88% over the predicted time frame. The market is expected to increase in value from US$ 24.61 Bn in 2022 to US$ 39.62 Bn in 2032.
The on US clinical trials Market, which provides a business strategy, research & development activities, concise outline of the market valuation, valuable insights pertaining to market share, size, supply chain analysis, competitive landscape and regional proliferation of this industry.
Download Free Sample@ https://www.precedenceresearch.com/sample/2480
A recent report provides crucial insights along with application based and forecast information in the Global US clinical trials Market. The report provides a comprehensive analysis of key factors that are expected to drive the growth of this market. This study also provides a detailed overview of the opportunities along with the current trends observed in the US clinical trials market.
A quantitative analysis of the industry is compiled for a period of 10 years in order to assist players to grow in the market. Insights on specific revenue figures generated are also given in the report, along with projected revenue at the end of the forecast period.
Companies and Manufacturers Covered
The study covers key players operating in the market along with prime schemes and strategies implemented by each player to hold high positions in the industry. Such a tough vendor landscape provides a competitive outlook of the industry, consequently existing as a key insight. These insights were thoroughly analysed and prime business strategies and products that offer high revenue generation capacities were identified. Key players of the global US clinical trials market are included as given below:
US clinical trials Market Key Players
- Parexel International Corp.
- Charles River Laboratory
- PRA Health Sciences
- Wuxi AppTec
- Eli Lilly and Company
- Novo Nordisk A/S
- Clinipace
- Omnicare
- Kendle
- Chiltern
Market Segments
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
By Indication
- Oncology
- Autoimmune
- Pain Management
- CNS Conditions
- Obesity
- Cardiovascular
- Diabetes
Report Objectives
- To define, describe, and forecast the global US clinical trials market based on product, and region
- To provide detailed information regarding the major factors influencing the growth of the market (drivers, opportunities, and industry-specific challenges)
- To strategically analyze micromarkets1 with respect to individual growth trends, future prospects, and contributions to the total market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of market segments with respect to four main regions—North America, Europe, Asia Pacific and the Rest of the World (RoW)2
- To strategically profile key players and comprehensively analyze their product portfolios, market shares, and core competencies3
- To track and analyze competitive developments such as acquisitions, expansions, new product launches, and partnerships in the US clinical trials market
Table of Content
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on US Clinical Trials Market
5.1. COVID-19 Landscape: US Clinical Trials Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. US Clinical Trials Market, By Phase
8.1. US Clinical Trials Market, by Phase, 2022-2032
8.1.1 Phase I
8.1.1.1. Market Revenue and Forecast (2022-2032)
8.1.2. Phase II
8.1.2.1. Market Revenue and Forecast (2022-2032)
8.1.3. Phase III
8.1.3.1. Market Revenue and Forecast (2022-2032)
8.1.4. Phase IV
8.1.4.1. Market Revenue and Forecast (2022-2032)
Chapter 9. US Clinical Trials Market, By Study Design
9.1. US Clinical Trials Market, by Study Design, 2022-2032
9.1.1. Interventional
9.1.1.1. Market Revenue and Forecast (2022-2032)
9.1.2. Observational
9.1.2.1. Market Revenue and Forecast (2022-2032)
Chapter 10. US Clinical Trials Market, By Indication
10.1. US Clinical Trials Market, by Indication, 2022-2032
10.1.1. Oncology
10.1.1.1. Market Revenue and Forecast (2022-2032)
10.1.2. Autoimmune
10.1.2.1. Market Revenue and Forecast (2022-2032)
10.1.3. Pain Management
10.1.3.1. Market Revenue and Forecast (2022-2032)
10.1.4. CNS Conditions
10.1.4.1. Market Revenue and Forecast (2022-2032)
10.1.5. Obesity
10.1.5.1. Market Revenue and Forecast (2022-2032)
10.1.6. Cardiovascular
10.1.6.1. Market Revenue and Forecast (2022-2032)
10.1.7. Diabetes
10.1.7.1. Market Revenue and Forecast (2022-2032)
Chapter 11. Global US Clinical Trials Market, Regional Estimates and Trend Forecast
11.1. U.S.
11.1.1. Market Revenue and Forecast, by Phase (2022-2032)
11.1.2. Market Revenue and Forecast, by Study Design (2022-2032)
11.1.3. Market Revenue and Forecast, by Indication (2022-2032)
Chapter 12. Company Profiles
12.1. Parexel International Corp.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Charles River Laboratory
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. PRA Health Sciences
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Wuxi AppTec
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Eli Lilly and Company
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Novo Nordisk A/S
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Clinipace
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Omnicare
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Kendle
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Chiltern
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 774 402 6168
Email: sales@precedenceresearch.com
Website: https://www.precedenceresearch.com


0 Comments