The high potency active pharmaceutical ingredients market would grow at a CAGR of 9.59% over the predicted time frame. The market is expected to increase in value from US$ 25.86 Bn in 2022 to US$ 53.8 Bn in 2030.
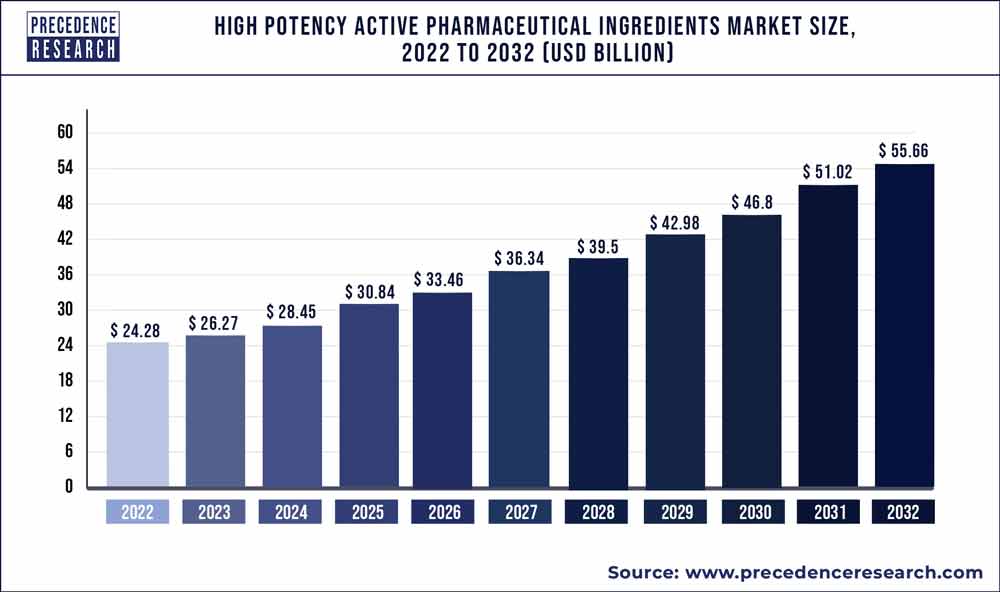
Key Takeaways:
- The North America market accounted for more than 36% of the total revenue share in 2021
- By manufacturer type, the in-house segment accounted 71% of the revenue share in 2021
- By application, the oncology segment has captured a market share of around 76% in 2021.
- By drug type, innovative drugs have accounted revenue share of over 71.6% in 2021.
- By product, the synthetic segment accounted revenue share of around 70.5% in 2021.
The on high potency active pharmaceutical ingredients Market, which provides a business strategy, research & development activities, concise outline of the market valuation, valuable insights pertaining to market share, size, supply chain analysis, competitive landscape and regional proliferation of this industry.
Download Free Sample@ https://www.precedenceresearch.com/sample/2324
A recent report provides crucial insights along with application based and forecast information in the Global High potency active pharmaceutical ingredients Market. The report provides a comprehensive analysis of key factors that are expected to drive the growth of this market. This study also provides a detailed overview of the opportunities along with the current trends observed in the High potency active pharmaceutical ingredients market.
A quantitative analysis of the industry is compiled for a period of 10 years in order to assist players to grow in the market. Insights on specific revenue figures generated are also given in the report, along with projected revenue at the end of the forecast period.
Companies and Manufacturers Covered
The study covers key players operating in the market along with prime schemes and strategies implemented by each player to hold high positions in the industry. Such a tough vendor landscape provides a competitive outlook of the industry, consequently existing as a key insight. These insights were thoroughly analysed and prime business strategies and products that offer high revenue generation capacities were identified. Key players of the global High potency active pharmaceutical ingredients market are included as given below:
High potency active pharmaceutical ingredients Market Key Players
- BASF SE
- CordenPharma
- Dr. Reddy’s Laboratories Ltd.
- CARBOGEN AMCIS AG
- Pfizer, Inc.
- Sun Pharmaceutical Industries, Ltd.
- Teva Pharmaceutical Industries Ltd.
- Albany Molecular Research, Inc.
- Sanofi S.A.
- Merck & Co., Inc.
- Novartis AG
Market Segments
By Product
- Synthetic
- Biotech
By Manufacturer Type
- In-house
- Outsourced
By Drug Type
- Innovative
- Generic
By Application
- Oncology
- Hormonal Disorders
- Glaucoma
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
Report Objectives
- To define, describe, and forecast the global high potency active pharmaceutical ingredients market based on product, and region
- To provide detailed information regarding the major factors influencing the growth of the market (drivers, opportunities, and industry-specific challenges)
- To strategically analyze micromarkets1 with respect to individual growth trends, future prospects, and contributions to the total market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of market segments with respect to four main regions—North America, Europe, Asia Pacific and the Rest of the World (RoW)2
- To strategically profile key players and comprehensively analyze their product portfolios, market shares, and core competencies3
- To track and analyze competitive developments such as acquisitions, expansions, new product launches, and partnerships in the high potency active pharmaceutical ingredients market
Table of Content
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on High Potency Active Pharmaceutical Ingredients Market
5.1. COVID-19 Landscape: High Potency Active Pharmaceutical Ingredients Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global High Potency Active Pharmaceutical Ingredients Market, By Product
8.1. High Potency Active Pharmaceutical Ingredients Market, by Product, 2022-2030
8.1.1. Synthetic
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Biotech
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global High Potency Active Pharmaceutical Ingredients Market, By Manufacturer Type
9.1. High Potency Active Pharmaceutical Ingredients Market, by Manufacturer Type, 2022-2030
9.1.1. In-house
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Outsourced
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global High Potency Active Pharmaceutical Ingredients Market, By Drug Type
10.1. High Potency Active Pharmaceutical Ingredients Market, by Drug Type, 2022-2030
10.1.1. Innovative
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Generic
10.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global High Potency Active Pharmaceutical Ingredients Market, By Application
11.1. High Potency Active Pharmaceutical Ingredients Market, by Application, 2022-2030
11.1.1. Oncology
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Hormonal Disorders
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Glaucoma
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Others
11.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global High Potency Active Pharmaceutical Ingredients Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Product (2017-2030)
12.1.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.1.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.4. Market Revenue and Forecast, by Application (2017-2030)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.1.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.1.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.1.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.1.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.6.4. Market Revenue and Forecast, by Application (2017-2030)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.4. Market Revenue and Forecast, by Application (2017-2030)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.6.4. Market Revenue and Forecast, by Application (2017-2030)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.7.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.7.4. Market Revenue and Forecast, by Application (2017-2030)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.8.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.8.4. Market Revenue and Forecast, by Application (2017-2030)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.4. Market Revenue and Forecast, by Application (2017-2030)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.6.4. Market Revenue and Forecast, by Application (2017-2030)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.7.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.7.4. Market Revenue and Forecast, by Application (2017-2030)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.8.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.8.4. Market Revenue and Forecast, by Application (2017-2030)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.4. Market Revenue and Forecast, by Application (2017-2030)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.6.4. Market Revenue and Forecast, by Application (2017-2030)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.7.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.7.4. Market Revenue and Forecast, by Application (2017-2030)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.8.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.8.4. Market Revenue and Forecast, by Application (2017-2030)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.5.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.5.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.5.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.5.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.6.4. Market Revenue and Forecast, by Application (2017-2030)
Chapter 13. Company Profiles
13.1. BASF SE
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. CordenPharma
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Dr. Reddy’s Laboratories Ltd.
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. CARBOGEN AMCIS AG
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Pfizer, Inc.
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Sun Pharmaceutical Industries, Ltd.
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Teva Pharmaceutical Industries Ltd.
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Albany Molecular Research, Inc.
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Sanofi S.A.
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Merck & Co., Inc.
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 774 402 6168
Email: sales@precedenceresearch.com
Website: https://www.precedenceresearch.com


0 Comments